Chemical reactions are essential. They put oxygen in our blood, salt in our food, and batteries in our phones. Find out why the periodical table is so brilliant.
Series: The Periodic Table and Sorting Elements – Part 2
Chemistry Happens
In Part 1 of this series, we looked at the difference between nuclear and chemical reactions. We saw that chemical reactions involve lower energies than nuclear reactions. That is fortuitous because the energy levels in most chemical reactions are low enough that we do not spontaneously disassemble as we would with nuclear reactions.
Chemical reactions are essential to biological processes ranging from hemoglobin carrying oxygen in our blood to our cocktail of acids, which aids us in digesting food. Chemical reactions form salt to flavor our food, steel, paints, batteries, power our muscles, etc.
Imagine how powerful it would be to have a key to predicting chemical reactions between elements quickly. I refer to a work of pure genius, the Periodic Table.
“The definition of genius is taking the complex and making it simple.”
― Albert Einstein, (attributed)
Systematic Simplicity
The Periodic Table of Elements is a systematic classification of elements by atomic number. It provides information about both nuclear and chemical attributes of each element.
In 1863, dissatisfied with the unwieldy descriptions of 56 known elements, Dmitri Mendeleev conceived a table to organize them. Mendeleev recognized the properties of elements and even left blank spaces where he felt elements remained to be discovered. His work was based on his observations of the weights of elements and their valence [1]. Without knowing the nuclear composition of atoms, he still developed an appropriate method to categorize nuclear and chemical properties.
Learn more about elements: The Secret Lives of the Elements
Why does Mendeleev’s Periodic Table Work?
If Mendeleev did not know about protons and neutrons in the atomic nucleus, why does his Periodic Table work? Mendeleev understood the relationship between atomic weights and valence electrons, which gave him an indirect understanding of protons in the nucleus. Hydrogen was the lightest element known, and it had one electron, so it became Element 1 on the Periodic Table. He then proceeded through the known elements sorting by weight and electrons. Mendeleev recognized properties that repeated in a predictable, periodic pattern related to an element’s number of electrons. Thus, he was able to leave gaps for elements, which were unknown at the time. He could even make predictions about the properties of the undiscovered elements.
Mendeleev labeled an unknown element “Eka-Aluminum” [Ea] because it would fill a gap in the same column as Aluminum but one row down.[2] In 1871 he predicted Eka-Aluminum would have an atomic mass of 68 (actual 69.7), a density of 6 gm/cm³ (actual 5.9), a low melting point (actual 29.8°C), and compounds with Oxygen [Ea2O3] or Chlorine [Ea2Cl3]. Gallium was discovered in 1875 and matched Mendeleev’s predictions – substitute Ga for Ea in the compounds mentioned above.
The Periodic Table is Your Friend
Chemistry becomes simpler to understand with the Periodic Table.
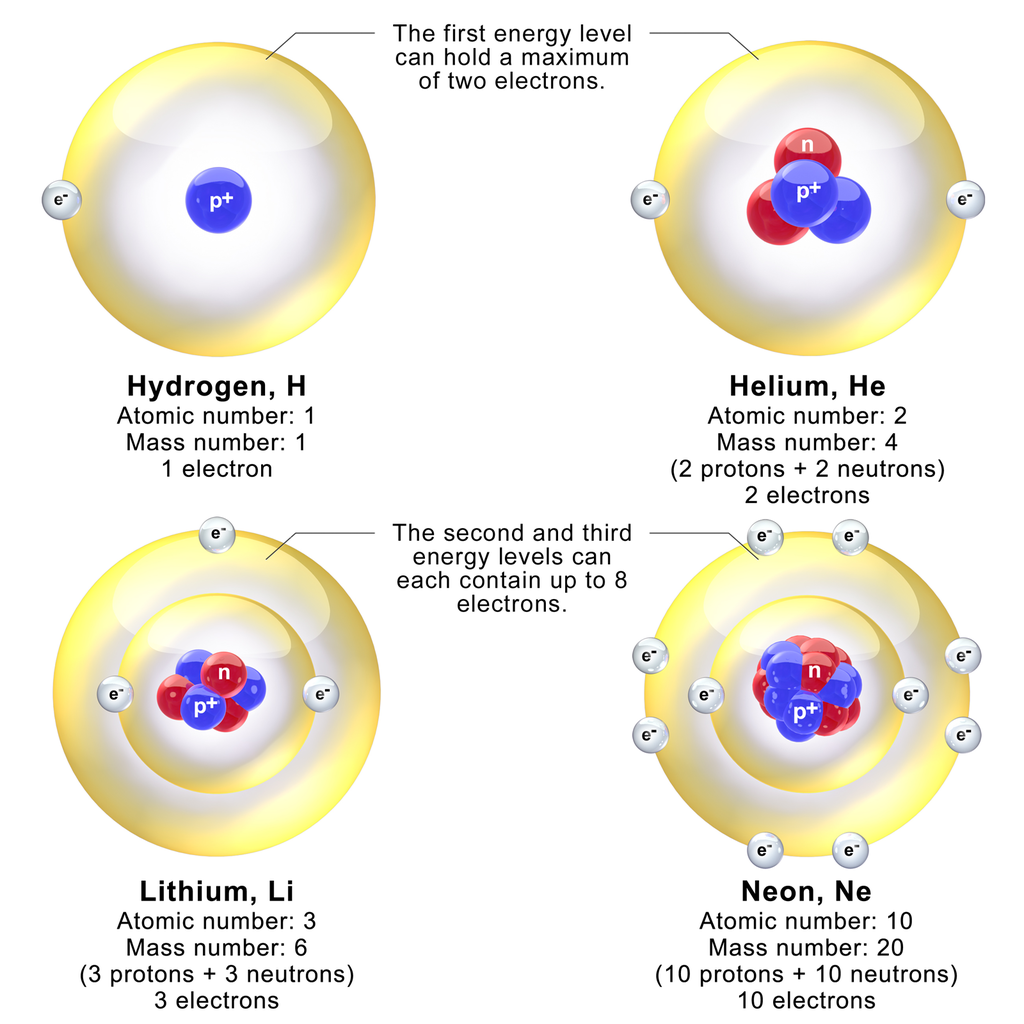
- The atomic number of an element is the number of protons in the nucleus; Hydrogen 1, Helium 2, Lithium 3, etc.
- The atomic weight reflects the isotopes [3] of an element and their relative occurrence. Iron’s atomic weight is 55.85, while its atomic number is 26. Even though the actual distribution of isotopes may be unknown to you, the atomic weight being fractional will indicates that there must be more than one isotope of iron. It is easy to determine that there must be at least isotopes of Iron [Fe] weighing 55 [Fe; 26 Protons + 29 Neutrons] or 56 [Fe; 26 Protons + 30 Neutrons] by subtracting the number of protons from the integer weights below or above the fractional number. NB: there are, in fact, more isotopes than this ranging from Iron-54 to Iron-60.
- Atomic sizes are largest in column one of the Periodic Table.
The smallest sized atoms are in those with a complete valence shell (typically, this is s2p6, however in Period 1, there is only s2, which is why Helium behaves like a “noble gas”).
Size increases with each row (Period).
Therefore, the smallest atom is Helium (Row 1, Column 18 of the standard Periodic Table), while the largest atom is Francium (Row 7, Column 1).
- The increase in size from row to row is due to the increased number of protons and neutrons in the nucleus as well as the corresponding increase in the number of electrons.
- The decrease in size from left to right across the table’s columns is due to how open the shells are, esp. the s- and p- sub-shells in the valence shell. This generally correlates with electronegativity. NB: (Sub-)shells will be discussed below.
- Electronegativity, which is the sharing of electrons in a bond between atoms, is predictable based on the location of an element in the Periodic Table. Why? Because there is a direct relationship between how full sub-shells are and how electronegative an element is. For students, think of it as how eager an element is to form a compound.
Electronegativity is especially useful in combination with the octet rule. The octet rule says that elements bond to make their valence shells full like a noble gas. Noble gases have full s- and p- sub-shells. Since s sub-shells have space for 2 electrons and p sub-shells have space for 6 electrons, a noble gas has 8 electrons in its valence shell.
As an example of a chemical bond following the octet rule, let us take a molecule of ordinary table salt (NaCl).
Chlorine (Cl) has 5 of 6 electrons in its 3p sub-shell filled. It needs one more electron to complete its valence 3-shell. Therefore, Cl is very “eager” to find an electron to fill the shell. It holds on to its electrons tightly because the shell is almost complete.
Chlorine’s sub-shells look like this: 1:s2 2:s2p6 3:s2p5
Sodium (Na) has 1 electron in its 3s sub-shell filled. It would need 7 more electrons to complete its valence 3-shell. The sole electron in the 3-shell is loosely held.
Sodium’s sub-shells look like this: 1:s2 2:s2p6 3:s1
Both elements can find configurations with a full octet if Sodium(Na) and Chlorine(Cl) combine. NaCl, table salt, is the result.
Here are their respective sub-shells when they combine:
- Na 1:s2 2:s2p6 << the outer shell has 8 electrons – it gives up its 3: s1 electron to Chlorine in the molecule, leaving it with a full valence 2-shell. Octet rule satisfied.
- Cl 1:s2 2:s2p6 3:s2p6 << the outer shell has 8 electrons – it has gained the 3: s1 electron from Na and filled the 3p sub-shell. Now its valence 3-shell is full. Octet rule satisfied.
- “Noble” Gases were termed noble because they remain unreactive (“aloof” or “pure”). They do not usually combine with other elements to form molecules, although this is possible. Why? They have full valence shells (s2p6). See the octet rule above.
- Many other qualities are hidden within the Periodic Table. Olympic medals are Bronze, Silver, and Gold. This is no coincidence. Although the Greeks had no precise, detailed insights into atomic nuclei, they recognized Bronze (Copper + Tin + optionally other metals) as valuable. They put it in the same series of precious metals with Silver and Gold. This grouping was very astute and accurately reflected similarities between Copper, Silver, and Gold. Examples: they are excellent conductors of heat, they have low electrical resistance, they are malleable, and have been used for coins or jewelry for millennia. You will find them on the Periodic Table in Column 11, Rows 4, 5, and 6.
She Sells Seashells, but I See Sub-Shells
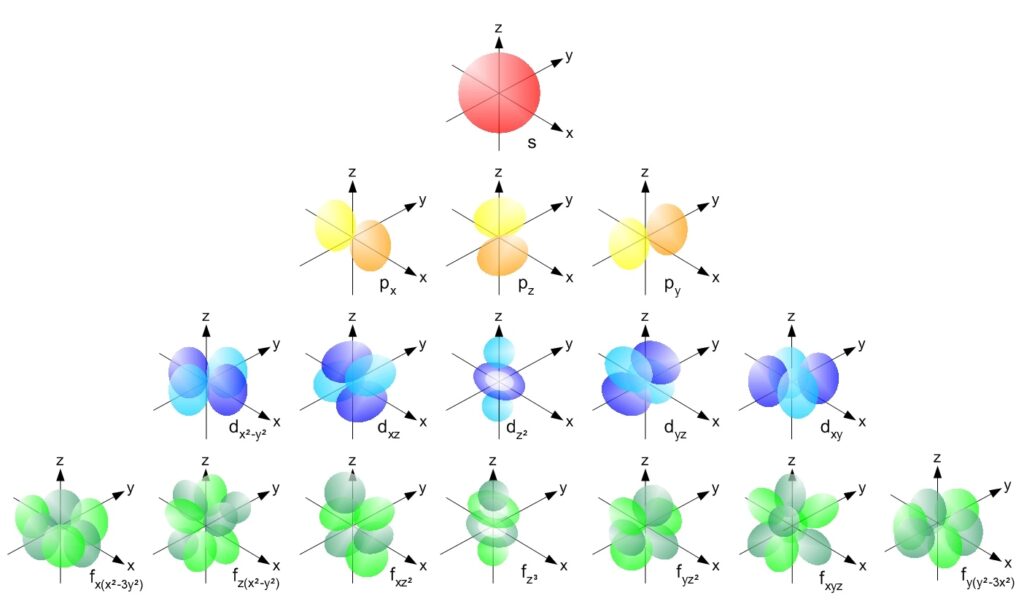
Shells help understand the chemical behavior of elements. There are sub-shells[4] as follows:
- The s sub-shell. It is completel when there are two (2) electrons in it.
- The p sub-shell may contain up to six (6) electrons.
- The d sub-shell may contain up to ten (10) electrons.
- The f sub-shell may contain up to fourteen (14) electrons.
- A theorized g sub-shell containing up to eighteen (18) electrons. (No element has been detected yet which has a g sub-shell, but the synthesis of such an element may be possible).
A Period ends when all the sub-shells in the Period have been filled.
Conclusion
The Periodic Table is a powerful learning aid. It ingeniously reflects underlying nuclear structures in the atomic number (protons) and the atomic weight (protons + neutrons). The number of electrons corresponds to the number of protons. Periods (rows) provide a repeating structure. The Groups (columns) in a Period are organized in blocks related to electron sub-shells. When all the sub-shells in a Period are filled, the element behaves as a “noble” gas, i.e., it typically does not bond with other atoms.
Many chemical traits can be anticipated based on the column and row. Examples include the size of atoms, electrical resistance, the conductivity of heat, and electronegativity.
With minor tweaks, the commonly used 18-column representation of the Periodic Table could be a more consistent and more powerful tool for students to understand the principles behind chemistry. A suggested version accompanies <Part 3> for the reader’s consideration.
Read the Series
The Difference Between Chemical and Nuclear Reactions
Pull up a chair and sit down to the Periodic Table and Sorting Elements – Part 1: The Difference Between Chemical and Nuclear Reactions.
Chemical Reactions: Periodic Genius
Chemical reactions are essential. They put oxygen in our blood, salt in our food, and batteries in our phones. Find out why the periodical table is so brilliant.
Find out why the Periodic Table of Elements is systematically brilliant. Also, get two free periodic tables that you can download and print for your classroom or dining room wall.
Endnotes
- Valence is defined by the International Union of Pure and Applied Chemistry (IUPAC) as “The maximum number of univalent atoms (originally hydrogen or chlorine atoms) that may combine with an atom of the element under consideration, or with a fragment, or for which an atom of this element can be substituted.” Essentially, in chemistry, valence is the number of bonds available in the outer shell of electrons minus the number of non-binding electrons. Hydrogen has an s-shell with one electron present. The s-shell may contain a maximum of two electrons. Hydrogen has a valence of one and can combine with other atoms to form compounds. Helium has two electrons in the s-shell. The shell is complete, so it does not react with other atoms chemically normally.
- Eka- from Sanskrit “one”. Mendeleev used it to describe an element one Period (row) below the known element.
- Isotopes: differing weights of an element. The element always has a fixed number of Protons, but the number of Neutrons may vary. As an example, Hydrogen is 1 Proton + 0, 1, or 2 Neutrons. We call Hydrogen with a Neutron Deuterium and Hydrogen with two Neutrons Tritium because they are two or three times the weight of “normal” Hydrogen (0 Neutrons).
- If you wonder (as I did) why the sub-shells are labeled s, p, d, and f, it is due to an obsolete method of categorizing spectral lines as “sharp,” “principal,” “diffuse,” or “fundamental.” Sub-shells beyond f will follow the alphabet, i.e., the next sub-shell will be the g sub-shell with 18 electrons. The h sub-shell with 22 electrons follows, the i sub-shell with 26 electrons, “j” will be skipped due to similarity with the letter “i”, the k sub-shell with 30 electrons, and so on. Synthesis of elements may not ever progress far enough to need a shell beyond the g sub-shell due to the energies required. Furthermore, some atomic nuclei and electron orbits calculations indicate that a limit may be reached at hypothetical Element 173. Search for Dirac equations and the Bohr model if you wish to learn more.
Additional Information
Excellent interactive Periodic tables may be found at:
- National Center for Biotechnology Information. “PubChem Periodic Table of Elements” PubChem, https://pubchem.ncbi.nlm.nih.gov/periodic-table/.
- Royal Society of Chemistry, Periodic Table” https://www.rsc.org/periodic-table
- Ptable https://ptable.com/?lang=en#Properties